Optimum Nanoparticles Design (ONPD)
Assisting Coordinated Progress in Longevity Science
Coordinate and accelerate global longevity R&D through a collaborative platform that bridges researchers, funders, and innovators - eliminating barriers, scaling impactful projects, and ensuring efficient resource use.
What is ONPD?
The nanomedicine R&D landscape - like much of biotech - is under pressure: Public funding is shrinking, venture capital is retreating, and supply chains are strained. Researchers face scattered data, incompatible materials, and costly IP barriers. Progress is stalling, not for lack of ideas, but lack of integration.
The Optimum Nanoparticles Design (ONPD) Platform is a specialized digital infrastructure designed to overcome this. By connecting fragmented knowledge, aligning key players: researchers, suppliers, labs, IP holders - and enabling scalable development, ONPD transforms today’s bottlenecks into tomorrow’s breakthroughs.
It also addresses deeper systemic issues: the complexity, fragmentation, and high cost of designing or replicating effective nanoparticle-based biomedical solutions.
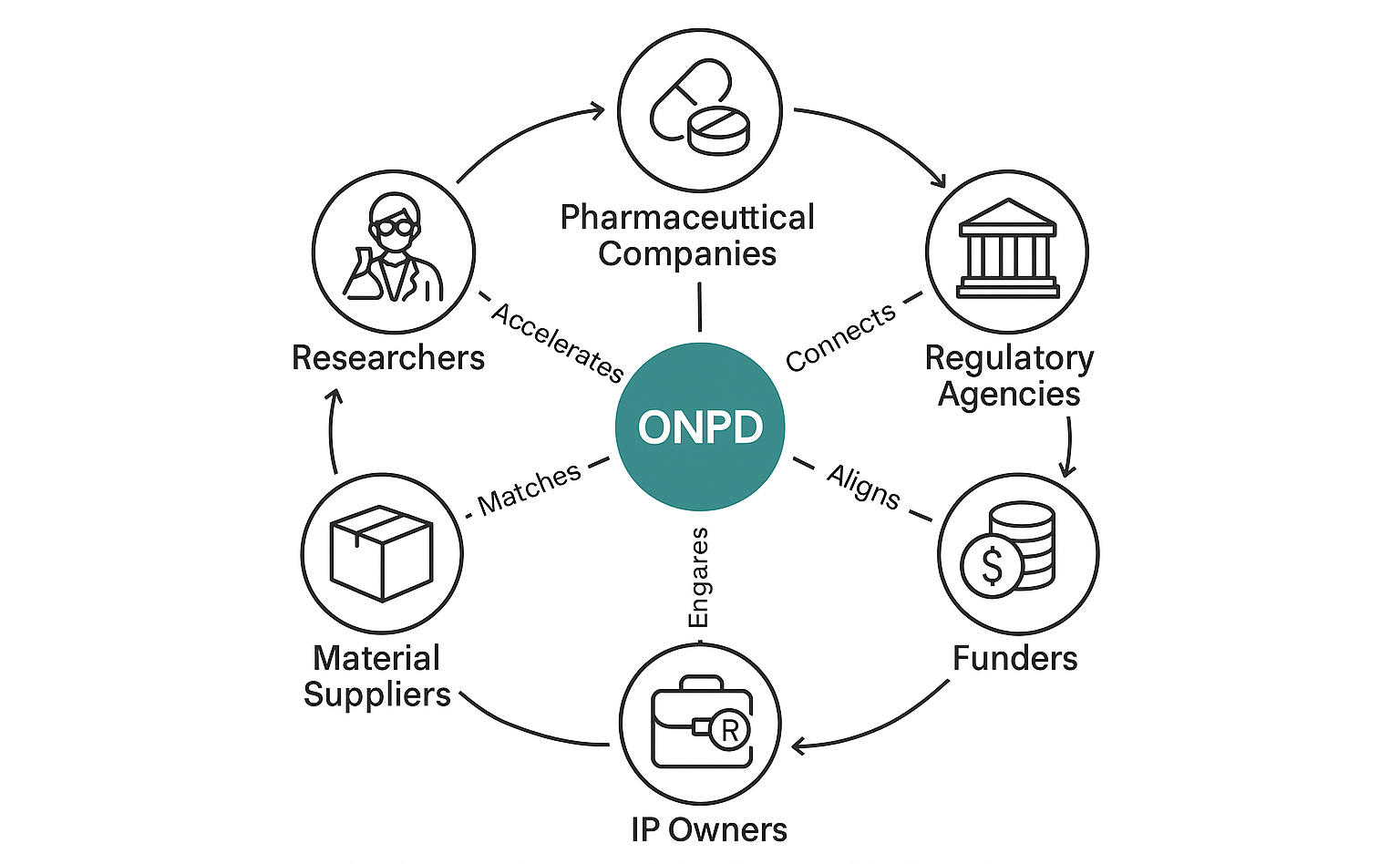
At the heart of the platform is a curated and expanding database of nanoparticles, each annotated with detailed physical, chemical, and biological characteristics; components; synthesis and testing protocols; associated intellectual property (IP) status and ownership (of the patented formulations), supplier information for materials/components; and laboratory protocols. Researchers working on novel therapies can specify their desired nanoparticle features, and the platform matches them with the most suitable, feasible or already-developed nanoparticle options - saving time, reducing duplication, and ensuring compliance with existing IPs.
Users of the platform include nanomedicine and modern intervention researchers, public and private research labs, universities, material suppliers, IP owners, and laboratories offering synthesis or characterization services. Material suppliers can upload their products and price lists directly to the system, ensuring real-time access to updated procurement options. The platform also offers lab protocols and price estimations.
IP owners can register their patented nanoparticles, define usage terms (e.g., academic research, licensing for R&D, or commercial use), and engage with researchers interested in licensing or collaborating. Through an IP Owner Dashboard, they retain full control over how their IP is accessed or used. In return, they gain global visibility, monetization opportunities through licensing, and analytics insights on demand and usage patterns, all while ensuring legal protection and compliance.
Two core operational activities support the platform: onboarding users and managing brokering. Onboarding involves attracting and activating diverse users: researchers, material suppliers, labs, and IP holders - through guided interfaces, tailored content, verification procedures, and assisted data uploads. This ensures that each user group can contribute meaningful content to the platform, whether it's materials, protocols, or IP records. Brokering adds further value by facilitating material procurement for research projects. The platform acts as a smart intermediary: aggregating supplier offers, suggesting optimized sourcing paths, estimating costs, and - when needed - directly brokering materials with reasonable margins. This reduces procurement friction for users while enabling supplier participation at scale.
What is ONPD Portal?
A unified ecosystem for research, manufacturing, and regulatory compliance in the nanomedicine era.
Smart Design Tools
AI-assisted matching and design of nanoparticle formulations.
Protocol & Supplier Access
Validated synthesis protocols and integrated supplier database.
Scale-up & Manufacturing
Guidance for industrialization and mass production readiness.
Global Harmonization
Track regulatory alignment across key global regions.
Public Health Impact
Moving toward enabling access to advanced, personalized therapies worldwide.
Who ONPD is for?
Serving researchers, suppliers, IP holders, laboratories, manufacturers, and the pharmaceutical industry in designing, accessing, and scaling therapeutic and diagnostic nanoparticles.
Researchers
Access nanoparticle data, design tools, and protocols.
Manufacturers
Connect to scalable methods, material sources, and CMC guides.
Regulatory Experts
Track global compliance needs with harmonized pipelines.
Pharma Innovators
Explore delivery technologies for emerging therapeutics.
Suppliers
Showcase products directly to researchers and manufacturers with real-time pricing and demand analytics.
IP Owners
Monetize patented nanoparticle technologies through smart licensing and global visibility.
Capital Providers
Discover fundable innovations, assess nanoparticle readiness, and track development pipelines.
Translational Centers & TTOs
Bridge academic discoveries with industry adoption through exposure and licensing support.
CROs & CDMOs
Offer synthesis, testing, and scaling services to a targeted community of innovators.
Standards & Policy Bodies
Shape the future of nanoparticle innovation through harmonized protocols and best practices.
The Challenges ONPD Addresses
Despite incredible advances in nanomedicine, researchers and innovators face major roadblocks that slow down progress and increase costs. ONPD is designed to directly address these pain points.
Structural Challenges
- Fragmented and Uncoordinated Data: Research on nanoparticle types, properties, and behaviors is scattered across publications, patents, and isolated labs, with no unified strategy for collection, verification, or integration, leading to persistent gaps and missed connections.
- Reproducibility Crisis: Lack of access to exact materials, protocols, or IP leads to inconsistent results and duplicated efforts.
- Lack of standardization: Nanoparticle-specific standards for synthesis, characterization, biological testing, regulatory approval, and clinical evaluation remain limited or absent.
- Disconnected Stakeholders: Researchers, manufacturers, IP holders, and regulators often operate in silos.
- Procurement Bottlenecks: Identifying and sourcing precise precursors or compatible materials can be time-consuming and opaque.
- IP & Licensing Barriers: Researchers waste time or risk infringement due to unclear patent landscapes or lack of licensing pathways.
Emerging Pressures
- Funding Disruptions: Sudden NIH policy shifts and grant delays threaten continuity in nanoparticle research and innovation.
- Funding Drought: Venture capital for early-stage biotech and nanomedicine sharply declined in mid-2025, leaving many startups underfunded and unable to advance preclinical R&D.
- Supply Chain Disruptions: New 2025 U.S. tariffs on imported APIs, reagents, and equipment - especially from China and India - have disrupted global supply chains, increased production costs, and caused delays in drug and nanoparticle manufacturing.
Join the ONPD Waitlist
We're currently preparing to launch the Optimum Nanoparticles Design Platform. If you'd like early access or project updates, please email us directly at info@longevity.com.
We're taking extra care with your data and won't collect personal information through this site until our infrastructure is fully secured.
Already in Motion
ONPD is already in motion. We’ve completed an AI-driven market sensing report to identify unmet needs and future demands. Our core backend database - built to manage complex scientific data - is fully developed and ready for data entry through Django Admin. It’s designed as the foundation of an evolving AI system that will not only store and organize nanoparticle knowledge, but also learn from it to support smarter design, matching, and decision-making. Its growth will be powered by a structured data acquisition strategy - combining literature mining, expert contributions, and verified experimental results - to ensure the platform remains comprehensive, accurate, and up-to-date. The website is live and continuously evolving. We’re now focused on onboarding visionary co-founders, early contributors, and pilot users to accelerate the next phase.
Open Seats for Visionary Co-founders
We’re in the earliest phase of building ONPD, and open to visionary co-founders who can help shape both the product and the mission.
We especially welcome collaborators with backgrounds in:
- Full-stack development (preferably Django)
- Nanomedicine or nanoparticle-based R&D
- Scientific database structuring
- UX/UI design (optional)
If you're passionate about accelerating innovation in nanomedicine and want to co-create a meaningful platform from the ground up, we’d love to hear from you.
➤ Please email us at info@longevity.com and tell us a bit about yourself.
